Why This Food Allergy Breakthrough Changes Everything for Millions
The food allergy breakthrough we've been waiting for is here. After decades of relying on avoidance and emergency treatment, we now have the first FDA-approved medication that reduces allergic reactions to multiple foods at once.
Key Food Allergy Breakthroughs in 2024-2025:
- Omalizumab (Xolair) - First multi-food allergy drug approved by FDA
- Protects against peanut, milk, egg, wheat, and tree nuts
- 68% of patients tolerated 2.5 peanuts vs 6% on placebo
- Available for ages 1 year and older
- Zileuton Pre-Exposure Pill - Revolutionary prevention approach
- 95% protection in mouse studies
- Human trials now underway
- Could prevent reactions before they happen
- Memory B Cell Findy - Potential future cure
- Scientists found the immune cell that "remembers" allergies
- Two therapeutic paths: eliminate or reprogram these cells
This shift from pure avoidance to active treatment represents a fundamental change in managing food allergies. For the first time, families have real options beyond carrying EpiPens and reading every ingredient label.
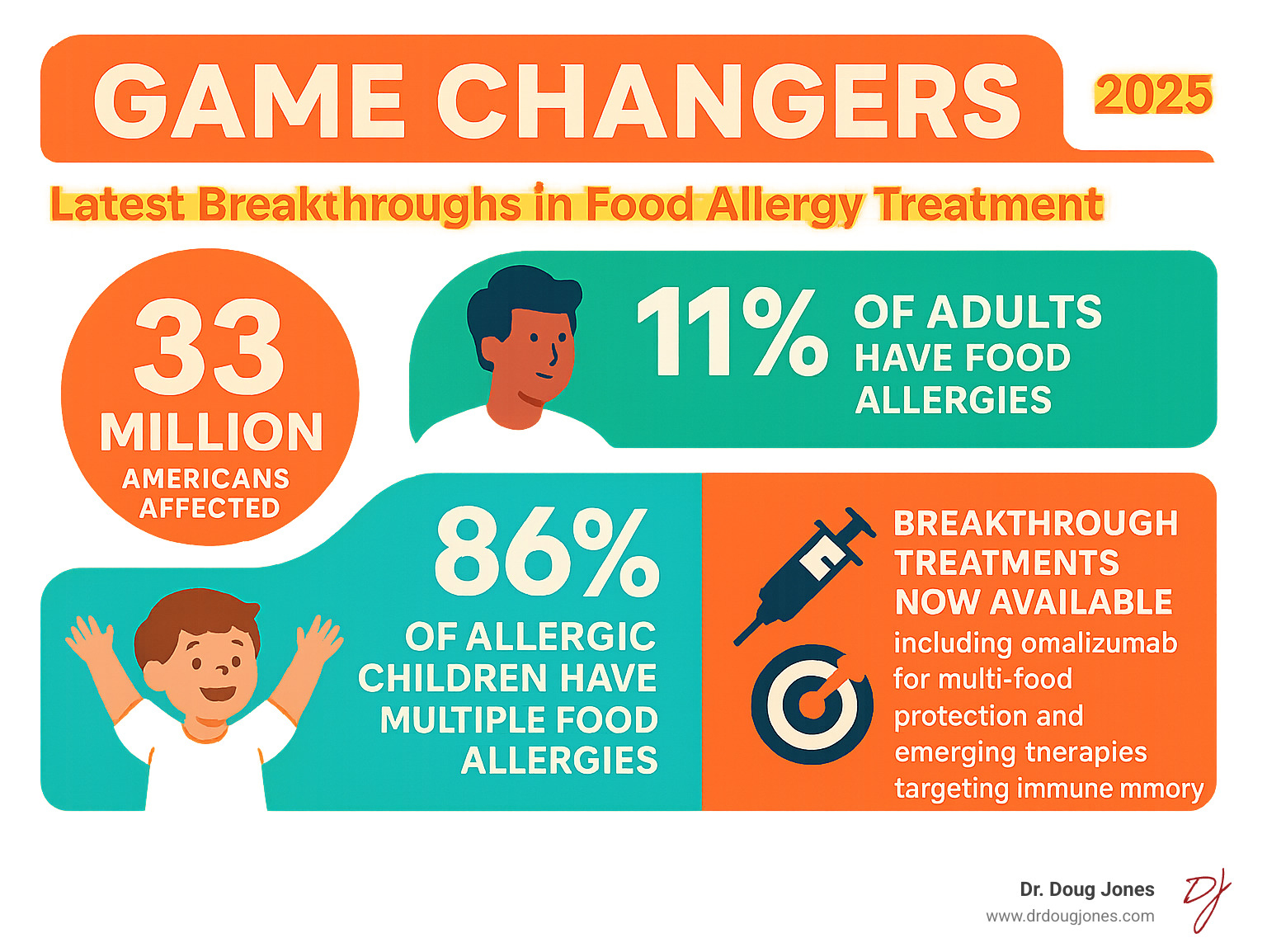
Food allergies now affect 33 million Americans—nearly 1 in 10 people. What makes these breakthroughs so significant is their ability to work across multiple allergens simultaneously. I'm Dr. Doug Jones, a board-certified immunologist who has spent over a decade treating complex immune conditions like food allergies. Through my practice at GAIN (Global Allergy Immune Network), I've witnessed how this food allergy breakthrough is changing patient care, and I help individuals steer these new treatments while addressing the root causes of their immune challenges.
The First Multi-Food Allergy Drug: A New Era of Protection
For decades, the only rule for food allergies was avoidance, a strategy that brought constant worry. The FDA approval of omalizumab (Xolair) marks a true food allergy breakthrough. It's the first medication to actively protect against multiple food allergies at once, creating a much-needed safety net.
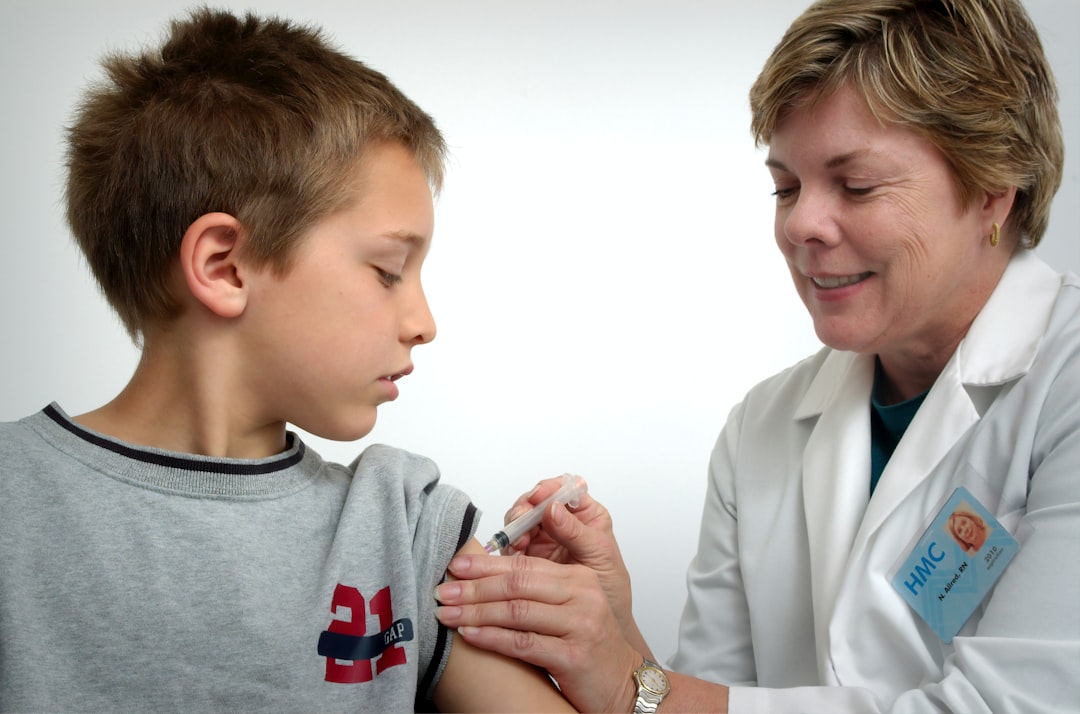
While not a cure, it offers real protection against accidental exposure, giving families an active defense system.
How Anti-IgE Therapy Works and Who is Eligible
In food allergies, the immune system produces IgE antibodies that treat harmless foods as threats. These antibodies attach to reaction cells, creating a hyper-sensitive alarm system where even trace amounts of an allergen can cause a severe reaction.
Omalizumab works by intercepting these IgE antibodies before they can arm the reaction cells, effectively turning down the volume on the allergic response. This raises the threshold for a reaction to occur. The key benefit is that it works for all food allergies simultaneously, raising the reaction threshold across the board.
Who is eligible? The FDA has approved omalizumab for individuals 1 year and older with confirmed IgE-mediated food allergies, verified by blood or skin prick tests. This is a game-changer for the 86% of allergic children with multiple food allergies, as it offers a single treatment for a complex problem.
You can dive deeper into the science behind this therapy in this scientific research on anti-IgE therapy for food allergy.
A Landmark Food Allergy Breakthrough: Recent Clinical Trial Results
Omalizumab's approval followed the impressive OUtMATCH clinical trial, which studied 168 participants with allergies to peanuts and at least two other common foods. The results after 16-20 weeks were remarkable: 68% of patients on omalizumab could tolerate at least 600 mg of peanut protein (about 2.5 peanuts) without a significant reaction, compared to only 6% on placebo.
This protection extended to other allergens:
- Milk: 66% of treated patients tolerated 1,000 mg of protein (vs. 11% on placebo).
- Egg: 67% were protected (vs. 0% on placebo).
- Cashew: 42% were protected (vs. 3% on placebo).
These numbers represent a new level of freedom from the paralyzing fear of accidental exposure. The 600 mg peanut threshold is especially meaningful as it covers most cross-contamination scenarios, significantly reducing daily anxiety even though intentional eating of allergens is still off-limits.
Anti-IgE Therapy and Other Approaches: What Sets It Apart
A common question is how omalizumab compares to oral immunotherapy (OIT), where patients consume increasing amounts of an allergen to build tolerance. The key difference is that OIT targets one specific food, while omalizumab protects against multiple foods simultaneously.
FeatureOmalizumab (Xolair)Oral Immunotherapy (OIT)Multiple Food ProtectionProtects against all your food allergies at onceUsually focuses on one food at a timeSafety ProfileVery low serious reaction rate (0% in trials)Higher reaction rate (over 30% in trials)Completion Rates88% of people completed treatmentOnly 51% completed treatmentDaily BurdenInjection every 2-4 weeksDaily food consumption requiredGoalProtection from accidental exposurePotential to intentionally eat the food
The OUtMATCH trial data underscores omalizumab's advantages in safety and tolerability. While OIT has its place, particularly for single-food allergies, omalizumab provides broader, safer protection for those juggling multiple sensitivities.
Practical Realities: Administration, Safety, and Precautions
Omalizumab is administered as a subcutaneous injection every 2-4 weeks, with the dose based on weight and IgE levels. Initial injections are given in a healthcare setting for monitoring due to a rare but serious boxed warning for anaphylaxis. After several safe doses, many patients can transition to self-administration at home, reducing the burden of clinic visits.
Common side effects are typically mild and include injection site reactions, fever, and headaches.
Crucially, omalizumab is not a license to eat allergens. Patients must continue strict avoidance and carry epinephrine. The treatment acts as a safety net against accidental exposure, not a cure. The real change is the significant reduction in daily anxiety. Knowing that a stray crumb or hidden ingredient is far less likely to cause a severe reaction allows families to participate more freely in school, travel, and social events. This food allergy breakthrough is about regaining quality of life while maintaining safety.
On the Horizon: The Next Wave of Food Allergy Research
While omalizumab represents a monumental food allergy breakthrough, scientists are pushing further, exploring new approaches that could make today's treatments look like just the beginning. Researchers are tackling food allergies from different angles, from pre-exposure pills to targeting the immune cells that "remember" allergies.
These aren't distant dreams—they're real research programs with promising early results that could bring even more dramatic changes in the next few years.
A Pill to Prevent Anaphylaxis? The Promise of New Pathways
Imagine a pre-exposure pill that could prevent a severe allergic reaction. Researchers at Northwestern University are testing this concept with a drug called Zileuton. Already FDA-approved for asthma, Zileuton was recently found to block a specific pathway (leukotriene/DPEP1) that triggers severe allergic reactions in the gut.
Mouse studies were remarkable: giving Zileuton before peanut exposure prevented allergic symptoms in 95% of mice. Lead researcher Dr. Stephanie Eisenbarth called the results "shocking."
This could provide an on-demand shield for high-risk situations like travel or dining out, supplementing—not replacing—epinephrine and avoidance. Human trials started in July 2025 to test its efficacy in people, including dosage and duration of protection.
You can read more about this groundbreaking research in Science. This represents a completely new way of thinking about food allergy protection.
Targeting the "Memory of Allergy": A Potential Future Cure
What if we could erase the immune system's memory of an allergy? This is the goal of cutting-edge research into a potential cure.
Scientists at McMaster University identified a special immune cell, the type-2 memory B cell (MBC2), as the body's "allergy memory bank." These MBC2 cells store the 'memory' of an allergy. When the allergen is re-encountered, they rapidly trigger the production of IgE antibodies, causing a reaction.
Now that these cells are known, researchers see two main approaches: eliminating these memory cells entirely or reprogramming them to not trigger dangerous reactions.
This research is still in early stages, but it represents a potential path to a true cure. Instead of managing allergies for life, we might one day be able to erase them. You can learn more about this promising research in this article about the newly identified immune cell and its role in food allergies. Understanding how our bodies "remember" allergies is a crucial step toward making a cure happen.
Understanding Your Treatment Options: From Desensitization to Remission
Navigating the landscape of new food allergy treatments can feel overwhelming. It's not just about what's available, but what each treatment aims to achieve. Working closely with your allergist is key to choosing a path that fits your goals, lifestyle, and expectations.

Defining Success: What Do These Terms Really Mean?
In food allergy treatment, terms like desensitization, remission, and tolerance represent different levels of success. Understanding them is crucial for interpreting results.
Desensitization is a temporary state of protection achieved during active treatment, like with omalizumab or OIT. The increased tolerance to an allergen typically fades if treatment stops. The recent food allergy breakthrough with omalizumab achieves desensitization for accidental exposures.
Sustained Unresponsiveness (SUI) is a more durable state where tolerance to an allergen persists for a period after treatment has stopped. It's a step beyond desensitization but not necessarily a permanent cure.
Remission implies a long-lasting state where the food can be eaten freely without reaction, similar to its use in other chronic diseases.
True Tolerance is the ultimate goal: a fundamental, permanent change in the immune system's response, where the food is no longer seen as a threat. This is effectively a cure.
Omalizumab primarily aims for desensitization, though early data suggests some patients might achieve SUI. Traditional OIT also targets desensitization, with hopes of achieving SUI or remission in some cases.
Evaluating Protocols Claiming Long-Term Remission
The idea of long-term remission is exciting, and some programs are making bold claims. For example, a specialized milk allergy protocol reported 100% remission in pediatric patients with a high safety profile, using AI to customize plans. While exciting, it's crucial to evaluate such claims thoughtfully.
When evaluating claims of long-term remission, consider:
- Study Rigor: Was it a randomized, controlled trial?
- Safety: What was the full picture of side effects and dropout rates?
- Repeatability: Can the results be replicated in other centers? Success may depend on specific protocols or patient selection.
- Durability: How long does the remission last? Longer follow-up provides more confidence.
New research into pathways that allow some people to tolerate foods despite positive allergy tests may help explain these outcomes and revolutionize how we diagnose and treat allergies, moving us closer to personalized, effective treatments.
Frequently Asked Questions about New Food Allergy Treatments
As families learn about these new food allergy breakthrough treatments, they naturally have many questions. Understanding the practical realities helps families make the best decisions.
Can patients stop avoiding foods after treatment?
The short answer is no, not yet. With omalizumab, you must continue avoiding known allergens. The treatment is approved to reduce reactions from accidental exposure, acting as a safety net, not a license to eat trigger foods.
However, ongoing research is promising. Early data from the OUtMATCH Stage 3 trial suggests many participants could eat small, daily amounts of their allergens after stopping treatment: 61-70% for milk, egg, or wheat, and 38-56% for peanuts and tree nuts. This suggests omalizumab may help build sustained unresponsiveness in some patients. Full results are expected in summer 2025.
How do I interpret "mg thresholds" in the real world?
Clinical trial thresholds can seem abstract, but they have real-world meaning. The 600 mg of peanut protein threshold (about 2.5 peanuts) tolerated in the OUtMATCH trial is a huge step for real-world protection.
Similarly, the 1,000 mg of protein threshold for foods like milk or egg represents a substantial exposure, far more than typical cross-contamination.
These thresholds matter in everyday situations involving potential cross-contamination—like at a school party or in a restaurant—which are a major source of anxiety. Being able to tolerate these amounts provides a large safety buffer. While you still can't intentionally eat allergens, the dramatic reduction in anxiety can be life-changing.
What are the cost and access considerations for these new treatments?
Realistically, cost is a significant factor. Omalizumab is an expensive biologic medication, often costing thousands per month without insurance. Insurance coverage varies, and navigating prior authorizations is common. Patient assistance programs can often help reduce out-of-pocket costs.
Initial clinic visits for injections and monitoring add to the cost and time commitment. However, the option for home self-administration after several doses can greatly reduce this burden.
Unfortunately, socioeconomic factors, insurance disparities, and geographic location can create barriers to access. While these treatments offer life-changing benefits, especially for toddlers and children with multiple food allergies, we must acknowledge these disparities. As these therapies become more established, access should improve for the 33 million Americans affected by food allergies.
Conclusion: Navigating a New Frontier in Allergy Care
We are at an exciting crossroads in allergy care. The recent food allergy breakthrough treatments—from omalizumab to research on Zileuton and memory B cells—signal a fundamental shift in managing food allergies.
For decades, the only tool was avoidance, which came at an enormous cost to peace of mind. Now, we are shifting to proactive management. We can raise reaction thresholds with omalizumab, may soon prevent reactions with pre-exposure pills, and are exploring ways to target the immune memory of allergies, opening the door to potential cures.
The journey isn't complete. Key questions about treatment duration and long-term protection remain, but researchers are actively seeking answers. Future milestones to watch in 2025 include the complete OUtMATCH Stage 3 results and early human data from Zileuton trials.
Navigating these new options underscores the importance of personalized care. Every person's allergy journey is unique, making an experienced guide invaluable.
As Dr. Doug Jones, I've dedicated my career to empowering individuals with complex immune challenges. Through our work at GAIN (Global Allergy Immune Network), we help you understand your unique immune landscape and create a personalized path forward. Whether that involves exploring these breakthrough treatments or addressing root causes, the goal is lasting relief and renewed freedom.
The future of food allergy care is bright. If you or someone you love is ready to explore what these advances might mean for your specific situation, we're here to help you steer this new frontier safely and effectively.
Find personalized allergy care




.png)